How many valence electrons does sodium have?
Can anyone explain how do we know how many valance electrons Sodium has. I mean not the answer but the process how to find the answer. Another question is why don’t we call it natrium. Thanks
3 Answers
-
In short, Sodium (Na) has one valence electron. How do you know it? Take a look at Mendeleev’s periodic table of elements and find Sodium (Na) in it.
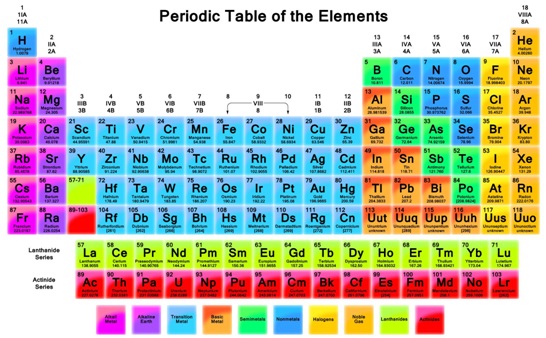
It’s in the first column and in the third row. And it means that Sodium has one valence electron. The number of the column is the same as the number of valence electrons in all the elements placed in that column.
-
Sodium (Na) is the eleventh element with a total of eleven electrons. The first 1s orbital can only hold 2 electrons, so the first two of them will be there. Then there is the 2s orbital, which can hold 2 electrons as well. So, the other two electrons go to 2s. Then we have 2b level, which can hold up to 6 electrons. Finally, the next level is 3s, where the remaining 1 electron goes. The electronic configuration of Sodium will be the following:
1s22s22p63s1
The electron on the last 3s level is the valence electron.
More detailed explanation is in this video:
-
Yes, Sodium has one valance electron. As to its name, the English-speaking world prefers the name Sodium over Natrium, while the rest of the world and the periodic table uses the word Natrium (Na). It’s all due to the history of the element discovery. It was first in 1807 that Sir Humphrey Davy discovered this element and named it Sodium. However, English was not lingua franca then. That is why when a few years later, the same element was rediscovered by a Swedish chemist Berzelius, he named it differently – Natrium (derived from Egyptian Natron – natural salt). Even though Davy was the first to discover the element, Berzelius was an international authority. The first periodic tables, including the English ones use the symbol Na for this element.


